All processes in plants are essentially chemical processes, more specifically biochemical, as they occur in living organisms. Many people view chemistry as something incomprehensible. Negative experiences often begin in school and are never corrected. However, if one wants to delve deeper into plants and their needs, some chemical insights are unavoidable.
A substance is everywhere and nowhere
The biggest challenge is probably accepting that a substance, a chemical element, can be found everywhere yet always appears different. When one element bonds with another, something new is created that has no similarities to the original materials. It seems as if the element has vanished, but in reality, it is still present, just in a compound with another element (which also seems to have disappeared).
Agricultural Chemistry
For example, let's consider the element carbon, which is the leader in forming compounds: There are known to be several million, with new ones being discovered daily. In contrast, the total number of compounds from all other elements is around 300,000. The three main forms of elemental, or unbound, carbon are diamond, graphite (pencil lead), and black coal, such as charcoal, activated carbon, or soot.
Humans are made up mostly of carbon compounds, along with water and bone minerals. The reason we don't appear black is due to a chemical principle: a substance in a compound has different properties than when it is alone or in another compound. However, when bodies are heated without air, the compounds break down and the carbon is revealed again; this process is called "carbonization".
From the list of carbon compounds mentioned earlier, it's clear that plants are heavily involved with this element. The carbon dioxide in the air is their primary source of "food", and along with water and light energy, they convert it into all the materials that make them up. Referring to fertilizers as plant nutrients is chemically inaccurate. Plants "feed" on carbon dioxide and water, and they need sunlight for this process, known as photosynthesis. If houseplants are kept in too dark an environment, they starve. Substances in the soil only play a role similar to minerals in our diet; they do not contribute to the "mass" of a plant.
At the same time, chemistry has discovered that substances do not "disappear" when they are no longer recognizable in a compound. They still exist, even if they cannot be seen, and when the compound is broken down, they re-emerge, as shown clearly by the charring of organic material. The carbon is back, likely transformed into carbon dioxide by decay bacteria, which the next green plant uses to create sugar that a person eats and incorporates into their body... an eternal cycle.
All elements behave similarly to carbon: they form various compounds and then seem to vanish. Crystalline substances (salts) contain metals, liquid water is made up of hydrogen and oxygen, and nitrates contain nitrogen, which in its elemental form is a crucial part of the air we breathe.
Norwegian author Jostein Gaarder has compared nature at this point to a box of Legos: the approximately 100 elements correspond to different building blocks, which are repeatedly assembled in various quantities into something new, then disassembled and used for something else. In this model, the chemical elements are like Lego bricks, from which you can build all sorts of things.
It is the job of soil bacteria to 'reconstruct' the available elements and compounds so that plants can absorb the necessary substances.
connects with another, something new emerges that has no similarity to the original materials.
In compounds, it becomes clear that we can no longer recognize carbon within them:
- The gases carbon dioxide, methane, propane,
- the liquids alcohol, gasoline, diesel, tar,
- the solids starch, cellulose, all sugars,
- proteins, all oils and fats, enzymes, dyes
... all of these are carbon compounds.
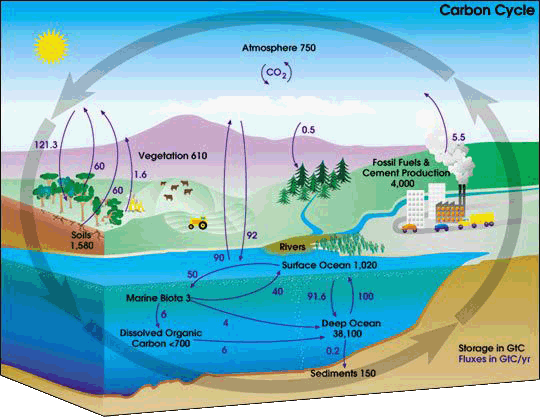
This carbon cycle diagram shows the storage and annual exchange of carbon between the atmosphere, hydrosphere and geosphere in gigatons - or billions of tons - of Carbon (GtC).
Burning fossil fuels by people adds about 5.5 GtC of carbon per year into the atmosphere.
Source: https://earthobservatory.nasa.gov/features/CarbonCycle
Author: User Kevin Saff on en.wikipedia