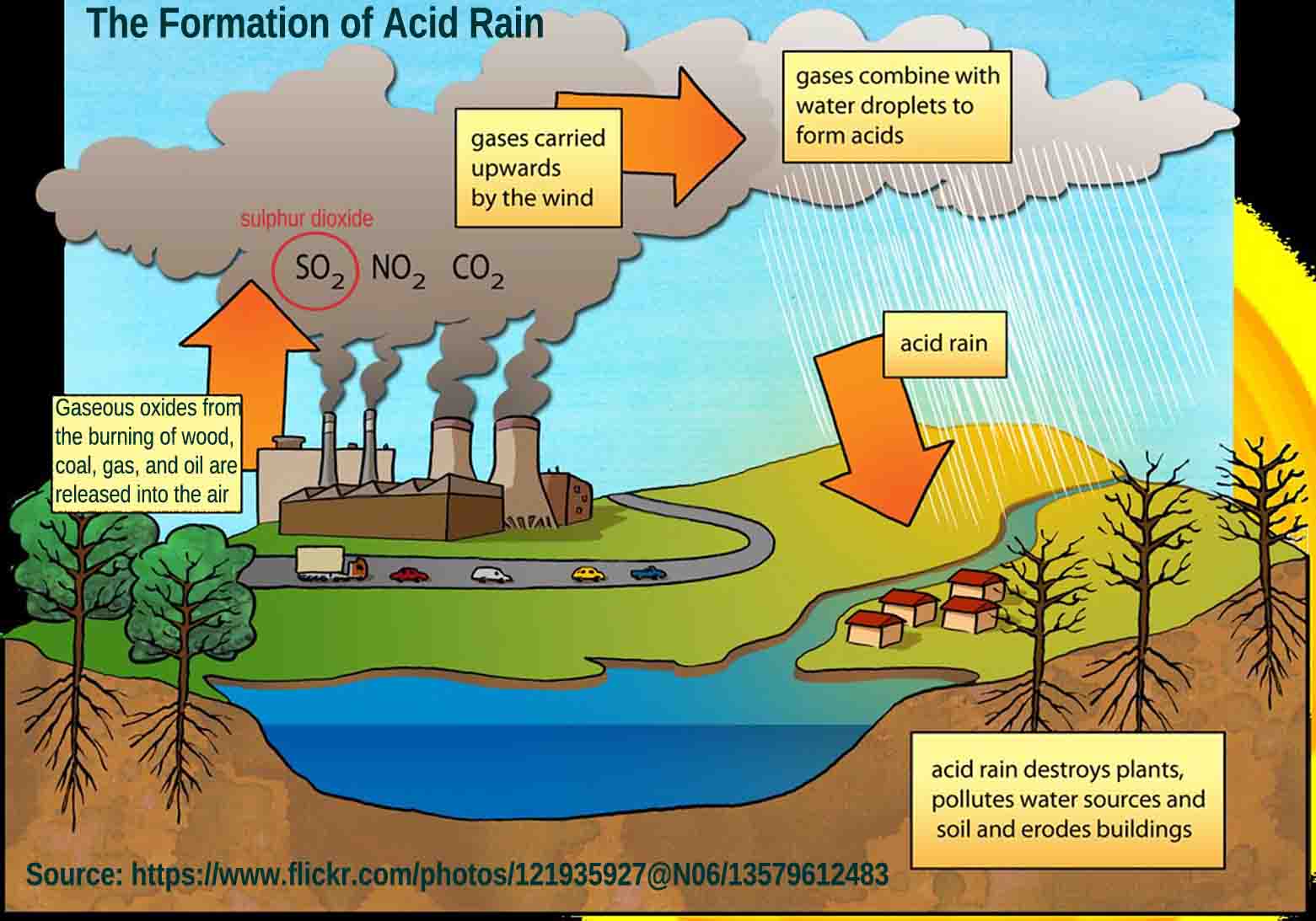
For the past 200 years, there was no need to fertilize with sulfur because the industrial chimneys and car emissions released so much sulfur dioxide that it caused damage as "acid rain" (for example, holes in nylon umbrellas). Therefore, it was unnecessary to spread sulfur-containing fertilizers; on the contrary, lime was sometimes used to intentionally neutralize the sulfur input.
This has been different since 20-30 years. Working desulfurization plants combined with a thriving gypsum production, which processes sulfur waste, have nearly eliminated sulfur dioxide from our air. Farmers were the first to notice this, and now sulfur is regularly included in agricultural "complete fertilizers."
However, private gardeners, especially those practicing organic farming, have not yet recognized the need for sulfur fertilization. To this day, sulfur is not mentioned as a fertilizer substance in gardening books!
Sulfur
Plants need sulfur for protein synthesis, which is the "living" substance in every cell. Without sulfur, there is no plant life! The mustard oils in onion plants and the spiciness of chili are also sulfur compounds. The biological breakdown of sulfur compounds is easy to identify: by the smell. The odor of rotten eggs, manure, feces, flatulence, etc., comes from sulfur breakdown products. This shows how often it is present in the nutrient cycle.
Especially the German University of Applied Sciences Weihenstephan conducted extensive research and gardening field trials on the use of sulfur at the beginning of the century. Unfortunately, the reports are no longer available online, even though they are still protected by copyright. Much of my knowledge comes from the reports published at that time. (Jauch, Martin und Schmitz, Heinz-Josef, Schwefel wirkt! pH-Absenkung bei Kompostsubstraten, Fachhochschule Weihenstephan: Forschung aktuell Mai 2004)
Sulfur can be applied in two ways:
- Industrial fertilizers contain it as sulfate, a water-soluble compound. Unfortunately, this leaches quickly into groundwater and can cause burns if misapplied. Soil bacteria are not engaged and may decline.
- As elemental sulfur, the only mineral permitted in organic farming. It is partly made from ground sulfur deposits from volcanoes. Elemental sulfur is not water-soluble. It must be converted by soil bacteria into a form that plants can use.
Sulfur bacteria are found everywhere in healthy soil. However, they only function within a narrow pH range; if the soil becomes too acidic, they stop working; when the acidity decreases, they resume processing sulfur. Therefore, over-fertilization is not possible. They can also eliminate (harmful) amounts of lime by forming compounds that turn lime into insoluble gypsum. Weihenstephan mentions in the above article that they can buffer up to 34% lime from natural compost.
Until a few years ago, one had to spread elemental sulfur as a powdered yellow substance, which was difficult to distribute especially in wind. Now, there are granulated sulfur fertilizers that consist of 80% sulfur and 20% rock flour, providing trace elements and contributing to humus formation.